RESEARCH
Microbial colonization on and inside plants
The plant is colonized by a plethora of microorganisms such as fungi, bacteria, archaea, oomycetes, and viruses that are detrimental or beneficial for their hosts (Hardoim et al. 2015; Brader et al., 2017). Some of these microorganisms colonize the plant surfaces before to enter inside plant tissues and form assemblages of subcommunities in various organs. Each step of the colonization of some of these microorganisms has been studied using single cells or natural communities. Different fluorescence techniques such as the use of tagged strains, fluorescence in situ hybridization or general staining techniques coupled with confocal microscopy have enabled to determine the colonization of these microorganisms (Compant et al., 2010). As an example, fungal colonization of a pathogen associated with grapevine has been revealed in Pierron et al (2015) and results have shown in which the niches the pathogenic strain could be present.
For bacteria, tagging of strains have been also done and a thorough understanding of the niches and ways of colonization of some specific plant-associated bacteria has been revealed (Compant et al., 2010; Escobar Rodríguez et al., 2018).
Different microbes have been detected crossing from different plant surfaces to inner tissues. Using general staining or specific targeting FISH, natural communities have been also visualized.
Colonization of root surfaces of corn by a gfp-tagged bacterium.
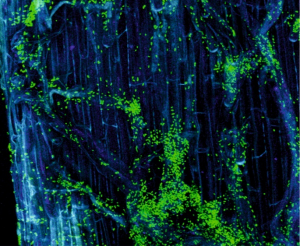
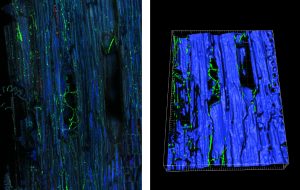
Colonization of a gfp-tagged fungus inside grapevine trunk assessed by confocal microscopy (left) and 3D modelling (right). From Pierron et al. (2015) and unpublished.
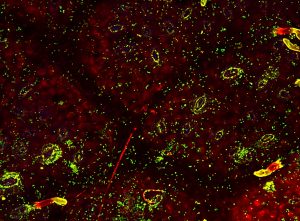
Bacteria (as green fluorescent and stained with syto9) on the phyllosphere of oak (from Vacher et al., 2016).
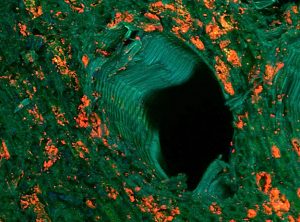
Actinobacteria (orange fluorescent) surrounding xylem vessels of grapevine trunk and assessed by FISH microscopy.
References
Brader G, Compant S, Vescio K, Mitter B, Trognitz F, Ma L, Sessitsch A. Ecology and genomic insights of plant-pathogenic and -nonpathogenic endophytes. Annual Review of Phytopathology (2017) 55: 61-83.
Compant S, Clément C, Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants. Their role, colonization, mechanisms involved and prospects for utilization. Soil Biology and Biochemistry (2010) 42: 669-678.
Escobar Rodríguez C, Mitter B, Barret M, Sessitsch A, Compant S. Commentary: seed bacterial inhabitants and their routes of colonization. Plant and Soil (2018) 422: 129-134.
Hardoim Pr, Van Overbeek Ls, Berg G, Pirttilä Am, Compant S, Campisano A, Döring M, Sessitsch A. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiology and Molecular Biology Reviews (2015) 79: 293-320.
Pierron R, Gorfer M, Berger H, Jacques A, Sessitsch A, Strauss J, Compant S. Deciphering the niches of colonisation of Vitis vinifera L. by the esca-associated fungus Phaeoacremonium aleophilum using a gfp marked strain and cutting systems. Plos One (2015) 10: E0126851.
Vacher C, Hampe A, Porté Aj, Sauer S, Compant S, Morris Ce. The phyllosphere: microbial jungle at the plant-climate interface. Annual Review of Ecology, Evolution, and Systematic (2016) 47: 1-24.
Leaf surfaces and their interaction with microbes
The first step in leaf colonization, pathogenic or beneficial, is adhesion of the respective diaspore preventing it from being removed by wind or rain. Adhesion of bacteria and fungal spores is a highly significant topic for agricultural research due to the economic interest in crop-pathogen interactions and in colonization with plant growth promoting bacteria and microbes for biocontrol.
Atomic force microscopy (AFM) has been applied in a few studies on the texture of leaf surfaces. Mechaber et al. [1996] was the first to use AFM for recording leaf texture, finding striking differences in roughness of young and old leaves of Vaccinium macrocarpum. Bargel et al. [2006] imaged molecular steps on the surface of single wax crystals with AFM, such steps are virtually undetectable by SEM. And in Koch et al. [2004] wax crystal formation on leaves of various species at the molecular level was shown for the first time with an AFM time-series under environmental conditions in vivo.
We performed a comparative study of Vitis vinifera Linne subsp. vinifera leaves of cultivars important for the Austrian wine producers in order to learn more about their surface architectures in the micro- and nanoscale [Konlechner and Sauer 2016].
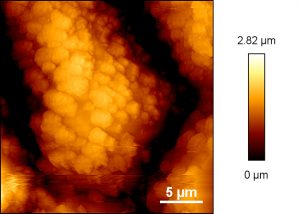
AFM image of epicuticular waxes on a grapevine leaf (variety Uhudler).
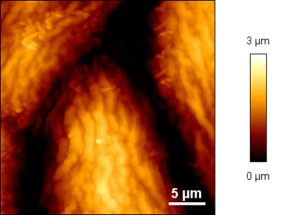
AFM image showing bacterial colonization on a grapevine leaf (variety Welschriesling).
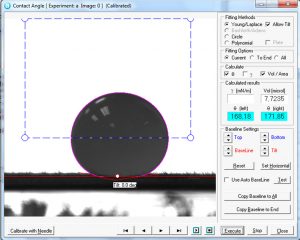
Drop of water on a young maize leaf.
The knowledge of wetting properties of leaf surfaces advance the insight in the interaction with additives, promoting the secure and optimal use of plant protection agents applied by spray deposition, especially under difficult conditions (climate). The aim of such research is better contact and or penetration, better adhesion of pesticides and other plant protecting agents but also improved adhesion of plant promoting bacteria in biocontrol applications.
Fungal spores for example can adhere to plant surfaces with pre-formed glycoproteins of the spore coat (e.g. Botrytis cinerea), with mucilage from the spore tip (e.g. Magnaporthe grisea) or by active secretion of adhesive glues (e.g. Peronospora parasitica) [Newey 2007]. For the investigation of the adhesion of fungal spores plant leaves and polystyrene has been used as substrate for adhesion assays [e.g. Newey 2007, Zelinger 2006].
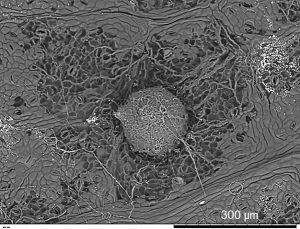
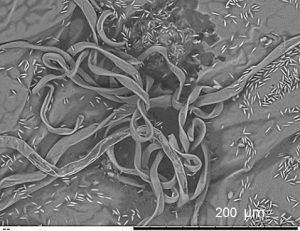
Grapevine leaf and fruiting body of the fungal pathogen Pseudopezicula tracheiphila.
Grapevine leaf and spores of the fungal pathogen Phomopsis viticola.
References
Mechaber WL, Marshall DB, Mechaber RA, Jobe RT, Chew FS. Mapping leaf surface landscapes. PNAS (1996) 93:4600-4603.
Bargel H, Koch K, Cerman Z, Neinhuis C. Structure-function relationship of the plant cuticle and cuticular waxes – a smart material. Functional Plant Biology (2006) 33:893-910.
Koch K, Neinhius C, Ensikat H-J, Barthlott W. Self assembly of epicuticular waxes on living plant surfaces imaged by atomic force microscopy (AFM). J Exp. Bot. (2004) 55(397):711-718.
Konlechner C and Sauer U (2016) Ultrastructural leave features of Austrian grapevine cultivars (Vitis vinifera L. ssp. vinifera). OENO One, 50(4):195-207.
Newey LJ, Caten CE, Grenn JR. Rapid adhesion of Stagonospora nodorum spores to a hydrophobic surface requires pre-formed cell surface glycoproteins. Mycological research (2007) 111: 1255-1267.
Zelinger E, Hawes CR, Gurr SJ, Dewey FM. Attachment and adhesion of conidia of Stagonospora nodirum to natural and artificial surfaces. Physiol Mol Plant Path (2006) 68:209-215.